The pharmaceutical distribution supply chain is the route a medication takes from the time it is manufactured to the time it reaches the pharmacy shelf. While consumers may not be aware of the distribution network, this path involves many steps prior to the time a consumer receives the medicine. U.S. consumers are fortunate to have a distribution supply chain that is among the safest in the world, yet increasingly sophisticated bad actors continue in their efforts to infiltrate the system. Implementation of the DSCSA will help strengthen the supply chain.
How the Supply Chain Works
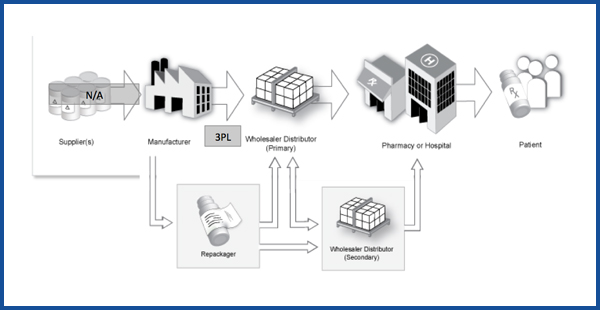
When a finished drug or biologic completes the packaging process at a manufacturing facility, its journey to a patient is just beginning.
For most products, the next leg of the journey takes them to a facility controlled by a wholesale distributor. Manufacturers may also contact with Third-Party Logistics Providers (3PLs) to coordinate the logistics.
Wholesale distributors, including primary and secondary distributors, are responsible for maintaining the integrity of medicines from the manufacturer to the dispenser, which distributes the medicine to patients. Distributors are the critical link between hundreds of manufacturers and more than 200,000 dispensers throughout the country.
Dispensers include hospitals, long-term care facilities, healthcare clinics, physician offices, and, of course, pharmacies, some of which may be small, independent drug stores, and others of which may be part of a chain of drug stores.
The DSCSA Established a Single, Uniform, and National Solution
On November 27, 2013, President Obama signed into law the Drug Quality and Security Act (DQSA), P.L. 113-54 (27 Stat. 587). P.L. 113-54 contains two separate titles: Title I addresses drug compounding and is known as “the Compounding Quality Act,” and Title II is known as “the Drug Supply Chain Security Act” (DSCSA). The DSCSA enhances the security of the pharmaceutical supply chain by establishing a national system for tracing and serializing pharmaceutical products and for establishing national licensing standards for wholesale distributors and third-party logistics providers. The DSCSA is a huge step forward in reducing potential threats to supply chain security and patient safety associated with pharmaceutical distribution.
For the full text of the DSCSA, please review the Public Law.